Describe effect of genetic variation on the pharmacokinetics
Question 1: Initial observations suggesting the presence of polymorphic CYP-mediated oxidative metabolism were based on inter-individual differences in an observed characteristic (e.g. drug concentration or clearance). Many early studies to characterize inter-individual variability in drug metabolism involved large population studies in which subjects were classified on the basis of function (i.e., enzyme activity) as being “poor metabolizers (PM),” “intermediate metabolizers (IM),” “extensive metabolizers (EM),” and in some cases “ultrarapid metabolizers (UM).”
Briefly define each of these terms with respect to genotype and phenotype. Specify a CYP enzyme that exhibits these phenotypic traits. Draw a figure to illustrate the expected genotype-phenotype relationship.
Question 2: In vitro data suggest that carboxylesterase 1 (CES1) metabolizes sofosbuvir, an ester pro-drug used for the treatment of hepatitis C infection. Describe your prediction for how a genetic polymorphism in CES1 would impact the bioavailability of sofosbuvir.
Question 3: The discovery of theorphan nuclear receptor pregnane X receptor (PXR),also referred to as the steroid and xenobiotic receptor, providedinsights into the molecular basis of how specific drugs couldinduce pathways of clearance and enhance rate of elimination of drugs. Genetic variation has been identified in PXR and the figure below shows data from a study designed to evaluate the functional significance of the variant.
Figure. Hepatic mRNA expression of PXR
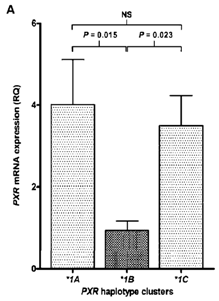
Question:Based on these data, list twodrug metabolizing enzymes that could be affected by this genetic variation. List the enzymes and predict how enzyme activity would be affected. Describe the mechanism by which this polymorphism could impact enzyme activity.
Question 4: Bicalutamide is an oral non-steroidal anti-androgen used in the treatment of prostate cancer.
A. Identify in humans an intestinal transporter that limits bicalutamideabsorption.
B. Briefly describe the pharmacogenetics of this transporter. List two common alleles, the allele frequencies, and include a comment for each SNP on whether it is functionally relevant (i.e., alters transport). Only discuss SNPs for which the frequency is >5%.
C. Describe the effect of the genetic variation on the pharmacokinetics of bicalutamide.
